Abstract
Background: CD19-specific 2nd generation chimeric antigen receptor (CAR) T cell therapy with either CD28 or 41BB co-stimulatory domain has demonstrated complete remission (CR) rates of 30-50% in relapsed or refractory (R/R) NHL and 20-30% in CLL. The lesser activity of CD19 CAR T cells in CLL and NHL relative to ALL may be in part due to diminished intrinsic T cell quality or greater CAR T cell inhibition in the tumor microenvironment. We previously demonstrated that 19-28z CAR T cells that express 4-1BBL achieve greater proliferation, IL-2 secretion and persistence compared to 19-28z or 19-4-1BBz CAR T cells (Zhao et al., Cancer Cell, 2015). Herein, we report on adult patients (pts) with CLL or NHL treated with escalating doses of autologous 19-28z/4-1BBL+ CAR T cells (NCT03085173).
Methods: Pts with R/R NHL (DLBCL, follicular lymphoma (FL), transformed FL (tFL), Waldenström's macroglobulinemia (WM)) and CLL including Richter's transformation were eligible for the trial. Pts received conditioning chemotherapy of cyclophosphamide (Cy) alone or combined with fludarabine (Flu) followed by escalating doses of CAR T cells. CAR T cells were administered at dose level (DL) 1 (1x105 CAR T cells/kg), DL2 (3x105 CAR T cells/kg), DL 3 (1x106 CAR T cells/kg), and DL4 (3x106 CAR T cells/kg). The primary objective of the study was to evaluate safety of autologous 19-28z/4-1BBLCAR T cells. Secondary objectives included rates of overall response.
Results: 25 pts were enrolled with R/R CLL (n=9), de novo DLBCL (n=6), tFL (n=3), FL and WM (n=4), and Richter's transformation (n=3). Median age of the pts was 70 (range, 53-81), and median number of prior treatments was 5 (range, 2-17). All 10 pts with CLL progressed on ibrutinib and 9 pts were refractory to venetoclax. 3 pts received DL1 CAR T cells, 3 pts DL2 CAR T cells, 3 pts DL3 CAR T cells and remaining 16 pts received DL4 CAR T cells. Of the 9 de novo DLBCL and tFL pts, all were >70 years old, 7/9 were refractory with progressive disease as best response to any line of chemotherapy or relapsed ≤12 mo after ASCT or any line of therapy. Six of 9 pts received R-GemOx as bridging chemotherapy with 2/6 progressing on R-Gemox prior to CAR T cell therapy. 3 pts received conditioning chemotherapy with Cy alone, and 22 pts received Flu + Cy conditioning chemotherapy. No dose-limiting toxicity has been observed.
Of the 25 treated pts, 24 pts are at least 2 weeks from T cell infusion and evaluable for toxicity. Overall, 16 pts experienced CRS (67%), all of which were grade 1 (n=13) and grade 2 (n=3). No pts experienced severe CRS. 8 pts (33%) experienced any grade neurotoxicity (NTX), including 6 pts with grade 1-2 and 2 pts with grade 3 NTX. No cerebral edema was observed, and all NTX were transient and reversible. 2 pts (8%) received one dose of tocilizumab and 4 pts (16%) received corticosteroid for NTX.
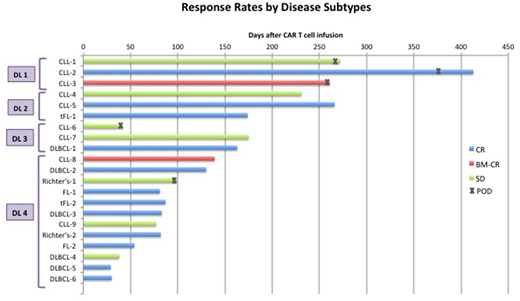
21 pts had at least 4 weeks of follow-up and were evaluable for response. 12 of 21 pts (57%) achieved CR, including 7 of 8 pts (88%) with large cell lymphoma, 2 of 2 pts with FL, 2 of 9 pts (22%) with CLL and 1 of 2 pts with Richter's transformation (Figure). CR was observed in pts with ≥10cm bulky lymph nodes (n=1), extra nodal disease involving bone, muscle, perineural and CNS (n=3), and extensive bone marrow involvement ≥80% (n=3). With a median follow-up of 93 days (range, 30 - 439), 11 of the 12 CR pts remain in CR.
Peak CAR T cell expansion occurred at a median of 9 days after CAR T cell infusion (range, 2-82). While analysis of CAR T cell persistence is ongoing, CAR T cell detection beyond 160 days has been noted.
Conclusions: Treatment with 19-28z/41BBL armored CAR T cells appears to be safe. No severe CRS was observed and severe NTX occurred in 8% of the pts with no case of cerebral edema. The overall CR rate of 57% is encouraging with 11 of the 12 pts remaining in CR at the time of this report. CR rates were higher in pts with large cell lymphoma (88%) compared to CLL (22%), though most of CLL pts received lower dose of CAR T cells (7 pts at DL1-3 vs. 2 pts at DL4). Pts with CLL may require higher doses of CAR T cells or incorporation of the CAR therapy in earlier lines of treatments. Detailed cytokine and CAR T cell expansion analysis in comparison to our previous cohort of pts treated with the 2nd generation 1928z CAR T cells will be presented.
Park:Adaptive Biotechnologies: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Shire: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; Pfizer: Consultancy. Palomba:Celgene: Consultancy; Pharmacyclics: Consultancy. Riviere:Fate Therapeutics Inc.: Research Funding; Juno Therapeutics, a Celgene Company: Membership on an entity's Board of Directors or advisory committees, Research Funding. Furman:Pharmacyclics LLC, an AbbVie Company: Consultancy; Acerta: Consultancy, Research Funding; Genentech: Consultancy; Gilead: Consultancy; Incyte: Consultancy, Other: DSMB; Loxo Oncology: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy; Janssen: Consultancy; AbbVie: Consultancy. Brentjens:Juno Therapeutics, a Celgene Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Sadelain:Fate Therapeutics Inc.: Research Funding; Juno Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal